Zoom link sent out to the department mailing list. For access to the link, please contact .
Abstract:
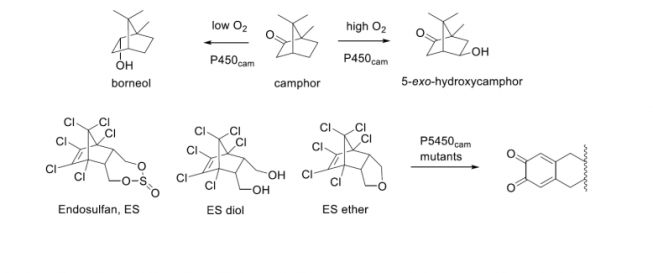
Cytochrome P450cam (CYP101A1) is well known for its catalysis of the hydroxylation of camphor, to give 5-exo hydroxycamphor. This is the first step in the degradation of camphor by Pseudomonas putida ATCC 17453, the strain from where P450cam and its redox partners were first isolated. The P450, a heme thiolate protein, requires a ferredoxin (putidaredoxin, PdX) and a PdX reductase (PdR) to couple the reduction of dioxygen to the oxidation of the 5-exo C-H bond of camphor. We have discovered that when O2 concentration is low, P450cam reduces a small amount of camphor to borneol and releases some reactive oxygen species. An investigation into the function of this unusual reaction revealed that borneol can shut down the expression of the P450cam/PdX/PdR system, which is under control of specialized repressor that is activated by camphor when O2 concentrations are high. We also found that P. putida ATCC17543 chemotax towards camphor and away from borneol. Our interpretation of this result is that camphor is toxic to bacteria, including this strain of P. putida, and it needs to be fully metabolized to not cause harm to the organism. The full biodegradation of camphor in P. putida requires 4 O2/camphor that is turned over by P450cam, which are not available when O2 levels are low. Investigation of the mechanism of this reaction revealed that P450cam needs to be in its closed conformation to produce borneol; a variant that is mostly open does not give this product. Mutations of key active site residues have revealed a tyrosine residue that participates in the reduction.
We have also randomly mutated P450cam and selected it on 3-chloroindole or on endosulfan, a persistent organic pollutant and formerly widely used insecticide. The mutants selected against 3-chloroindole convert that compound to isatin, a pink dye that is easy to identify. The mutants selected against endosulfan convert it and related compounds, such as endosulfan diol, ether or lactone, to ortho-diquinones, by way of an elimination cascade of all six chloro substituents found in these compounds. This is interesting, as endosulfan is only interconverted between various functional groups at the non-halogenated end in nature. Here we have discovered variants of P450cam that remove all the halogens from these pollutants.