
High Resolution NMR Spectroscopy
About

The Nuclear Magnetic Resonance facility offers research support as well as both solution and solid-state data services. We have a long track record of providing consultation to our users with the appropriate NMR methods and helping them to extract their desired structural or dynamic information. To discuss your NMR research projects or find out our current services, please contact the manager of the NMR facility:
Contact:
Zhicheng (Paul) Xia, Ph.D.
Office: (604) 827-3548
Lab: (604) 822-6787
Fax: (604) 822-2847
Email:
Our Members:
Dr. Zhicheng (Paul) Xia, NMR Facilities Manager
Tel: (604) 827-3548
Email:
Dr. Maria Ezhova, Research Assistant
Tel: (604) 822-6787
Email:
Pengfei Xu, Research Assistant
Tel: (604) 827-0796
Email:
Location:
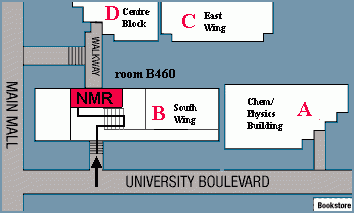
The primary NMR lab is located in room B460 in South Wing of the Chemistry Department at 2036 Main Mall, University of British Columbia. Other spectrometers are in B353, D126 and E120.
Instruments
Bruker Avance 300 Spectrometer
- Bruker QNP 5mm probe (auto-switchable quad nucleus probe for observing 1H, 13C, 31P, and 19F).
- BACS-120 autosampler
- VT range -150 to +180°C.
- All Standard 1D and 2D exps.
- Gradient/Shape pulse capability.
- Updated to Topspin 2.1 on Windows XP.

Bruker Avance 400inv Spectrometer
- Bruker 5mm BBI (inverse broadband probe with Z-gradient coil tunable from 109Ag to 31P).
- VT range -150 to +180°C.
- All Standard 1D , 2D experiments.
- 19F observe using proton coil (therefore can’t decouple 1H).
- Gradients/Shaped pulses.
- Updated to Topspin 2.1 on Windows XP.

Bruker Avance 400dir Spectrometer
- Bruker direct 5-mm z-gradient BBO with automatic tuning and matching.
- BACS-120 autosampler.
- VT range -150 to +180°C.
- All Standard 1D , 2D experiments.
- Shaped pulse capabliity.
- Low gamma probe 10mm coil for freq. below 109 Ag (property of Prof. L. Schafer).
- Updated to Topspin 2.1 on Redhat Linux WS 4.

BRUKER AVANCE 600 (with CRYOPROBE)
- Bruker z-gradient TCI (1H, 13C,15N) cryoprobe.
- Bruker 5mm z-gradient TXI (1H, 13C and 15N) as backup probe.
- All standard 2D experiments.
- 15N can be inversely detected.
- VT range is limited to +10°C to +50°C.
- Updated to Topspin 2.1 on Redhat Linux WS 4.

Bruker AVANCE III AV300 at SIF
- Bruker BBFO probe with ATMA.
- Z-gradient and shaped pulse capability
- VT range -150 to +180°C.
- All Standard 1D and 2D exps.
- Running Topspin 2.1 on Redhat Linux WS 4.
- This is primarily a teaching instrument.

Bruker Wide Bore Solid State AV400
- MAS triple channel broadband tunable probe with 4mm rotor.
- X-winnmr 2.6 on SGI O2
- This is a service-only spectrometer.

Bruker AV III HD 400 MHz
- Bruker BBFO smart probe with ATMA.
- Z-gradient and shaped pulse capability
- VT range -150 to +180°C.
- All Standard 1D and 2D exps.
- Running Topspin 3.2 on CentOS 5.

Services
Forms:
| Request for NMR Service |
| Request for NMR Training |
| Request for NMR Login |
Training Notes:
| Basic Training | Intermediate Training |
Spectrometer time reservation policies:
Online Booking:
Sample Submission:
We offer technician based service on AV400inv/dir. For exceptional needs we have limited ability to run experiments on AV600 as well - please discuss your experiments with NMR staff. In order to submit sample for the service, please bring prepared sample in NMR tube to room B460, and fill the requisition form. Your sample will be done on a “first come - first served” basis. We can process your data in the lab. and print a hard copy, or we can send your raw data via e-mail as a zipped file and you can process it on your own computer using XWIN-NMR or Mest-Rec, or other available software.
Sample preparation:
Preparation of the NMR samples has to be done in your own work space.We do not have facilities (fume hood, selection of the solvents, NMR tubes etc.) to do this in the NMR laboratory.
While preparing your NMR sample, please, pay attention to the following:
1. NMR tubes: Quality has to comply with requirement for your NMR experiment.
We are using 5 mm NMR tubes for all our spectrometers. High precision 5 mm NMR tubes should be used for AV600 cryoprobe and for high quality spectrum on AV400inv/dir. The “standard” NMR tubes had to be at least 15 cm long. The J-Young NMR tubes had to be at least 14 cm long before the valve. If you are using repaired J-Young tube, make sure the sealing is as good as possible. It will affect your shimming/resolution.
While you are choosing J-Young tube, be aware, that size of the valve does matter for our current setup (using string for lifting such tubes). Usually J-Young tubes with larger valves give more problems during locking/shimming, which result in poor resolution.
Cleaning NMR tubes could be done by rinsing with appropriate solvent. Scratching tubes should be avoided (use copper wire in case of absolute needs). If you are using base bath for cleaning, make sure that you remove all traces of base by washing with acidic solution, water and acetone. Do not dry NMR tubes in oven. Remember to wash new NMR tubes as well. They are not clean.
Links
Sigma Aldrich NMR tubes
Norell NMR tubes
2. Solvent: Choice of the solvent depends on the solubility of your sample, chemical shift of the residual protons in 1H NMR and position of signals in 13C NMR (position of the solvent peaks can overlap with signals from your compound), chemical (solvent should be inert towards your compound) and physical (b.p., m.p., viscosity need to be considered while doing VT NMR experiments) properties of the solvent.
Links
http://www.isotope.com/cil/products/listproducttypes.cfm?prodtypeid=41
3. Sample volume: 4.5 cm of solution should be used for different probes on AC200, AV300, AV400, AM400 magnets; exactly 4.0 cm of solution required for the cryoprobe on AV600. If you are limited by quantities of sample, submit sample for AV600 or you can use lower volume (~ 3.0 cm) of solution on other spectrometers. In this case you need to optimize position of the tube in the spinner turbine by centering volume of your sample relative to the centre marker on the depth gauge. If you are using larger volume (> 4.5 cm), do not center your solution, place NMR tube in spinner to a depth of 20 mm from the centre of coil. If sample volume is significantly different (larger or smaller from the optimum 4.5 cm depth) then either longer SHIMMING or GRADSHIMMING will be required to get good resolution, especially for proton spectra.
4. Concentration: For 1H spectra (high resolution Δ1/2 < 0.5 Hz) generally used <1 to ~40 mgs/0.4 ml depending on molecular weight. Please remember that:
i) dilute solution is better than concentrated since linewidth (Δ1/2) increases with increasing viscosity.
ii) chemical shift (δ) can vary due to solvent, or temperature, or concentration.
iii) high viscosity solvents should be avoided if really sharp spectra are desired (e.g. deuterated DMSO, benzene, pyridine, H2O, etc.)
For 13C spectra concentrations of 10-300 mgs/0.4 ml are common depending on MW. A few things to consider:
i) since natural linewidths are 5-10 times wider than in 1H spectra, viscosity is usually not a factor.
ii) Signal/Noise (S/N) proportional to concentration, but S/N also proportional √ #scans therefore it takes 4 times longer to run a 1 mg/ml sample as it does a 2 mg/ml sample for the same S/N.
Remember to: i) avoid layers of concentration in NMR tube, make sure its mixed; ii) run solvent blank NMR spectrum if sample very dilute.
5. Common impurities [PDF]
Web data transfer/processing
The whole nmr experiment(s) (not just the fid file) has to be transferred from the spectrometer computer (AV300, AV400inv, AV400dir, AV600cp) using FTP routine or sent by e-mail as a zipped file.
You can process these files by using XWIN-NMR (version 2.6, and 3.5) on our four work-stations (three work-station located in B460 and one located in A239) or by Mest-Rec or ACD processing software at your own computer.
If you are processing files transferred through FTP routine – work as you normally process it on the spectrometer computer. If you are working with zipped files – unzip it first.
If you are using Mest-Rec nmr processing software
- Open folder 1 in Mest-Rec 1D or 2D processing application and then highlight the fid file in folder 1, if a 2D exp file is opened then highlight the ser file which is the serial set of files for the 2D nmr experiment.
- Then process the raw data.
- To attach the acquisition info (i.e. exp. name, date, ns, sw, etc) you need to open the folder 1 again outside of Mest-Rec and go to the subfolder in 1 called pdata. Click on it and double click on the file called parm. It will probably open in MS NOTEPAD. Highlight the text file info you want to put on your spectrum and copy it to clipboard (i.e. ctrl C).
- Go back to your spectrum in Mest-Rec and click the A icon on the menu bar and do a paste (i.e. ctrl v) to right, center or left of the spectrum.
If you are processing files from AV300 or AV400inv using XWIN-NMR, you can print your files from XWIN-NMR or XWIN-PLOT. If you are processing files from AV400dir or AV600cp using XWIN-NMR, you can print your files from XWIN-PLOT only.
Referencing proton and carbon spectra should be done by using chemical shift of residual protons and chemical shift of 13C respectively from the solvent used to dissolve your sample. We recommend using the Table from Cambridge Isotope Laboratories [PDF] for the exact numbers. Referencing spectra of other nucleus should be done by using correspondent standard. We are currently using CH3NO2 (set at 0 ppm) or 15NH415NO3 (set at 0 ppm for NH4) for 15N NMR; CFCl3 (set at 0 ppm) or trifluoroacetic acid (set at -78.5 ppm) for 19F NMR; 85% H3PO4 (set at 0 ppm) and triphenylphosphate (set at -16.4 ppm) for 31P NMR.
Bruker Avance 600
Training NMR:
All new users of the NMR facility are required to take two 1.5 hr training sessions before being checked out by staff.
After a successful check-out, a username / password will be issued. Both INTRODUCTORY and ADVANCED sessions will be run on the AV300/400 spectrometers every few months.
Please sign up for these sessions on the posted sheet in B460 and/or by answering the email announcement. Students who sign up for these user training sessions should be ready to acquire and process NMR spectra as part of their research… otherwise procedures will be forgotten!!!
A basic understanding of FT NMR is essential. Good references to read over before sessions are found in chapters 1-3 of [REF 1] or the first chapter of [REF 2] or chapters 2-4 of [REF 3] ( See below). There will be copies of Ref 1 Chapter 3 in Rm. B460 just before scheduled training.
User Notes in PDF format are available for AV300, AV400, and AV600. These should help users go through the everyday routine on their own after the training sessions, as well as do more sophisticated experiments on all spectrometers.
REFERENCES:
1. Claridge,Timothy, ‘High-Resolution NMR Techniques in Organic Chemistry’, Pergamon Press 1999 Chapters 1-3. (a very good ref. text for the new NMR user!!)
2. Sanders, J. K. M., and Hunter, B. K., " ‘Modern NMR Spectroscopy’ A guide for Chemists”, 2nd Ed Oxford Press 1993 Chapter 1
3. Derome, A., ‘Modern NMR Techniques for Chemistry Research’ Pergamon Press 1987 Chapters 2-4.
Booking: