Performances of catalysts and more generally of functional materials is mainly controlled by our ability to generate specific sites/objects (organometallic complexes, nanoparticles, uniform thin films…) onto supports (powders or flat surfaces). In this context, we have developed a rational approach to yield functional materials containing well-defined surface-species.
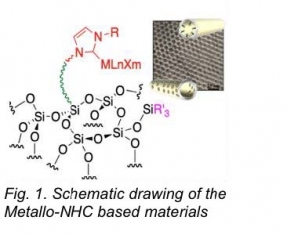
Two main areas of research will be presented here. First, we have developed synthetic methods allowing the preparation of well-defined functional materials containing either organic fragments, heterobimetallic complexes[1] or highly active metallo-N-heterocyclic carbene complexes (M-NHC). The materials were fully characterized using several techniques and particularly by advanced solid-state NMR using Dynamic Nuclear Polarization which allowed us to have a molecular description of surface species. Of the different materials, solids containing TEMPO radicals for MRI applications [2] and M-NHC (M = Ru and Ir) based catalysts [3] will be presented. The M-NHC catalysts exhibit enhanced catalytic compared to those of homogeneous homologues in solution.[3] These remarkable performances were attributed to surface site isolation which suppresses bimolecular deactivation processes and to beneficial interactions between the silica surface and the M-NHC centres.
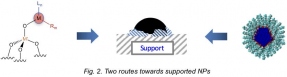
Second, we investigated the controlled grafting of late transition organometallic complexes onto silica powders to yield very small and calibrated metallic NPs and compared this methodology to solution protocols to prepare metallic NPs in solution.[4] Applications in hydrosilylation of olefins will be detailed.
[1] S. Lassalle et al. J. Catal. 2020, 392, 287; S. Lassalle et al. J. Am. Chem. Soc. 2019, 141, 19321
[2] M. Cavaillès, A. Bornet, X. Jaurand, B. Vuichoud, D. Baudouin., M. Baudin, L. Veyre, G. Bodenhausen, J.-N. Dumez, S. Jannin, C. Copéret, C. Thieuleux Angew. Chem. Int. Ed. 2018, 57,7453.
[3] M. Samantaray et al. J. Am. Chem. Soc. 2013, 135, 3193; M. Conley et al. ACS Catal. 2014, 14, 145; I. Romanenko et al. Angew. Chem. Int. Ed. 2015, 54,12937; M. Renom-Carrasco et al. Dalton
Transactions 2019, 48, 2886
[4] P. Laurent et al. Dalton Trans. 2013, 42(1), 238; D. Baudouin et al. J. Am. Chem. Soc. 2012, 134,
20624; T. Galeandro-Diamant et al. Chem. Commun. 2015, 51, 16194; T. Galeandro-Diamant et al. Chem. Commun. 2017, 53, 2962; T. Galeandro-Diamant et al. Catal. Sci. & Tech. 2019, 9, 1555; M. Jakoobi et al. J. Org. Chem. 2020, 85, 11732.
