Abstract:
The action of hydrogen peroxide on the copper-containing enzymes lytic polysaccharide monooxygenases (LPMOs) has been shown to enhance the activity of the enzymes on saccharidic substrates, but also lead to rapid inactivation of the enzyme, presumably through protein oxidation.
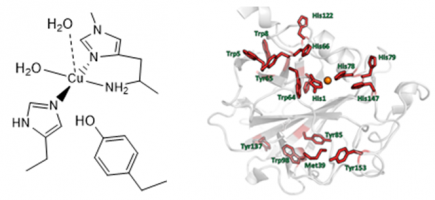
Figure: active site structure of an LPMO and oxidized amino acids (red) following treatment with H2O2.
Here, through the use of UV/vis, CD, XAS, EPR, MCD, MS and resonance Raman spectroscopies augmented with DFT calculations, we show that one of the products of protein oxidation in a AA9 LPMO is a long-lived ground-state singlet Cu(II)-tyrosyl species, which is inactive for the oxidation of saccharidic substrates. The formation of the stable Cu(II)-tyrosyl species requires the presence of a water molecule in the axial position of the copper coordination sphere, which then allows the d(x2-y2) SOMO to rotate towards the tyrosyl to form a short Cu-(OTyr) bond. The water molecule is only present in substrate-free conditions, meaning that the binding of substrate prevents Cu(II)-tyrosyl formation and thus protein inactivation during coupled catalytic turnover.
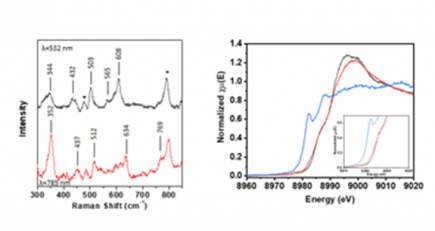
Figure, left resonance Raman spectrum of Cu(II)-tyrosyl, right Cu K-edge X-ray absorption species
References
[1] K.E.H. Frandsen et al, Nature Chemical Biology, (2016), 298-303.
[2] B. Bisarro, A.K. Rohr, G. Muller, O. Chylenski, M. Skaugen, Z. Forsberg, S.J. Horn, G. Vaaje-Kolstad, V.G.H. Eijsink, Nature Chemical Biology, (2017) 1123-1128.
