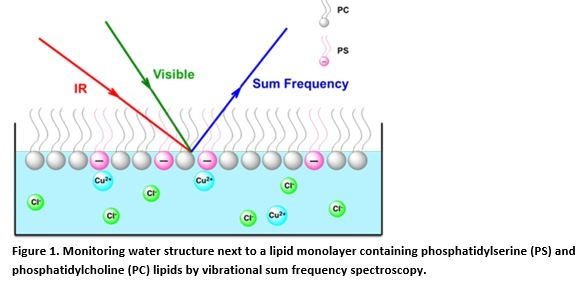
Abstract: Biological membranes often contain lipids with free amine groups like phosphatidylserine (PS) and phosphatidylethanolamine (PE). The head groups of these lipids typically only interact weakly with divalent metal ions such as Ca2+ or Mg2+. However, we have found that they interact far more strongly with transition metal ions like Cu2+. PE and PS lipids are highly regulated within cells and are highly abundant in certain organelles, while almost completely absent in others. Despite the high degree of control of lipid composition within cells, little is often known about the reason for it or even the specific nature of ligand-receptor binding interactions with metal ions. To remedy this, we have employed a combination of spectroscopic techniques (Figure 1), microfluidic platforms, monolayer and planar supported bilayer architectures to explore the specific biophysical chemistries of these interactions. This includes the development of a novel analytical tool that employs a dye-conjugated fluorophore that is extremely sensitive and selective toward the binding of Cu2+ ions. Both the thermodynamic and molecular level details of these interactions have been obtained. The results reveal that binding can be highly dependent on the concentration of PS or PE lipids within the membrane as well as solution pH. The potential physiological significance of these interactions will be discussed.