A pyridoxal phosphate-dependent enzyme that oxidizes an unactivated carbon-carbon bond
Enzymes use organic cofactors to facilitate diverse chemical transformations. One of these cofactors, pyridoxal phosphate (PLP), is best known for its ability to stabilize anions, which enables reactions such as transaminations, critical in the breakdown of amino acids. However, PLP is rarely known to react with oxygen or to catalyze reactions on unactivated carbons.
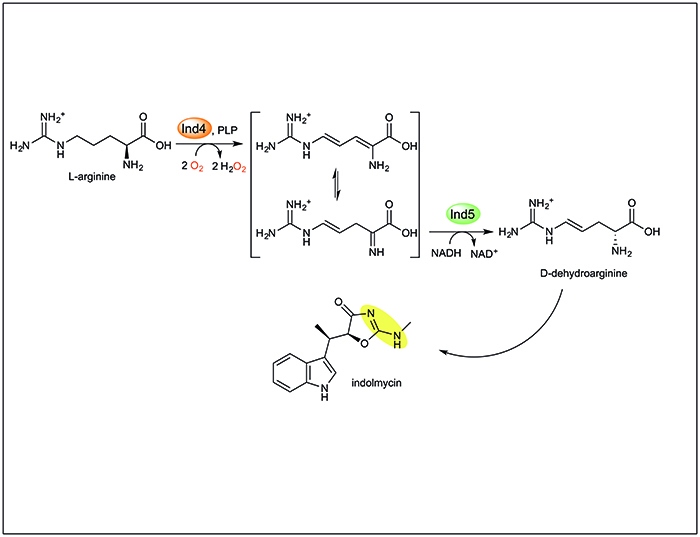
However, recent work from Katherine Ryan’s lab, published today in Nature Chemical Biology, will lead to a reevaluation of the power of this organic cofactor. The paper, “A pyridoxal phosphate-dependent enzyme that oxidizes an unactivated carbon-carbon bond,” reports the unexpected result that Ind4, an enzyme annotated as a “standard” PLP-dependent aminotransferase in the biosynthetic pathway to the antibiotic indolmycin, is capable of oxidizing an unactivated C-C bond in the amino acid arginine using molecular oxygen. These results will expand our understanding of the catalytic versatility of PLP-dependent enzymes and enrich the toolbox for oxidative biocatalysis.
The authors of this work are Yi-Ling Du (UBC Chemistry), Rahul Singh (UBC Microbiology & Immunology), Lona Alkhalaf (UBC Chemistry), Eugene Kuatsjah (UBC Genome Science + Technology), Hai-Yan He (UBC Chemistry), Lindsay Eltis (UBC Microbiology & Immunology), and Katherine Ryan (UBC Chemistry).
Link to article: http://www.nature.com/nchembio/journal/vaop/ncurrent/full/nchembio.2009.html