Abstract: Since mid-2011 researchers in our laboratories have been developing a process that we call the hexadehydro-Diels–Alder (HDDA) reaction.1 This net [4+2] cycloisomerization produces an o-benzyne derivative, which is then rapidly captured in a subsequent trapping event. The HDDA reaction is a rare example of a transformation that generates a highly reactive intermediate by way of a highly exergonic (ca. –50 kcal•mol-1) reaction! This two-stage, benzyne generation-trapping cascade results in the rapid assembly of structurally complex benzenoid products. This chemistry is both preparatively valuable and mechanistically enlightening. New modes of benzyne reactivity2,3,4 along with fundamentally new mechanistic understanding have been uncovered. In this lecture I will discuss more recent developments by focusing on three-component reactions in which two different trapping agents are incorporated into the final product.
The Hexadehydro-Diels–Alder (HDDA) Cascade:
– here involving two trapping reactions and, therefore, comprising 3-component reactions –
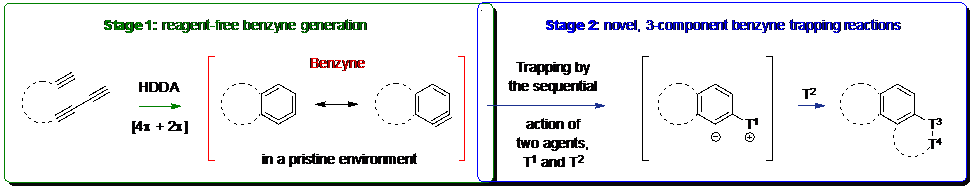
These will include:
• trapping initiated by sulfur nucleophiles5
• trapping initiated by nitrogen nucleophiles (including natural product derivatization6)
• the photochemical HDDA reaction7
• the aza-HDDA reaction (in the context of the pentadehydro-Diels-Alder reaction7)
• the domino-8 and bidirectional-HDDA reactions
1 The hexadehydro-Diels–Alder reaction. Hoye, T. R.; Baire, B.; Niu, D.; Willoughby, P. H.; Woods, B. P. Nature 2012, 490, 208–212. (doi:10.1038/nature11518)
2 Alkane desaturation by concerted double hydrogen atom transfer to benzyne. Niu, D.; Willoughby, P. H.; Baire, B.; Woods, B. P.; Hoye, T. R. Nature 2013, 501, 531–534. (doi:10.1038/nature12492)
3 The aromatic ene reaction. Niu, D.; Hoye, T. R. Nature Chem. 2014, 6, 34–40. (doi:10.1038/nchem.1797)
4 Mechanism of the reactions of alcohols with o-benzynes. Willoughby, P. H.; Niu, D.; Wang, T.; Haj, M. K.; Cramer, C. J.; Hoye, T. R. J. Am. Chem. Soc. 2014, 136, 13657−13665. (doi:10.1021/ja502595m)
5 Reactions of HDDA-derived benzynes with sulfides: Mechanism, modes, and three-component reactions. Chen, J.; Palani, V.; Hoye, T. R. J. Am. Chem. Soc. 2016, 138, 4318–4321.
6 Complex and diverse structures from reactions of HDDA-benzynes with natural products. Ross, S. P.; Hoye, T. R. Nature Chem. 2017, 9, 523–530. (link)
7 Photochemical hexadehydro-Diels-Alder (h-HDDA) reaction. Xu, F.; Xiao, X.; Hoye, T. R. J. Am. Chem. Soc. 2017, 139, 8400–8403. (link)
7 The pentadehydro-Diels–Alder reaction. Wang, T.; Naredla, R. R.; Thompson, S. K.; Hoye, T. R. Nature 2016, 532, 484–488. (link)
8 The domino hexadehydro-Diels-Alder reaction. Xiao, X.; Hoye, T. R. manuscript under review.
