Natural Product Synthesis
Our research focuses on the development of new methods for the syntheses of architecturally complex natural products. This encompasses the use of oxygen- and nitrogen-centered radicals for the synthesis of heterocycles, organometallic reagents for the development of new bond construction techniques, the enantioselective synthesis of building blocks for total synthesis, and the development and evaluation of proposed biogenetic pathways through biomimetic synthesis. Central to efficient methods development is a fundamental understanding of reactivity and reaction mechanisms. Reaction kinetics are, therefore, fully integrated with all methodology development to maximize the reaction's utility and efficiency.
Recent highlights of our research are:
- We have developed a new oxygen-centered radical cyclization onto silyl enol ethers to form versatile siloxy-substituted tetrahydrofurans, a key component of many bioactive compounds. These cyclizations have been found to be among the fastest radical cyclizations known. The increased cyclization reactivity allows cyclizations even when competing reactions are present, such as a cyclization onto a terminal alkene, 1,5-hydrogen abstraction, and beta-fragmentation. The increased reactivity also allows for the synthesis of tetrahydropyrans in substantially higher yields than any other oxygen-centered radical cyclization methodology.
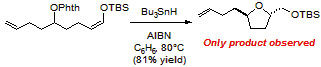
- We have developed an efficient method for the rapid construction of bioactive carbo- and heterocycles using radical relay cyclization initiated by alkoxy radicals. This new method allows for the rapid increase in molecular complexity from simple starting materials though the conversion of unactivated C-H bonds into reactive intermediates. The cyclizations are effective for the synthesis of a wide range of cyclopentane, pyrrolidine, tetrahydropyran, and tetrahydrofurans derivatives.
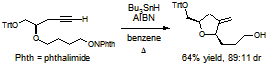
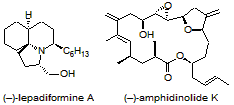
We are currently further exploring these new synthetic methods and utilizing them in the total synthesis of both (–)-lepadiformine A and (–)-amphidinolide K. Each synthesis involves synthesizing complex heterocyclic fragments by using sequential or cascade reactions in which complexity is rapidly achieved from simple linear precursors. We are also currently developing new, non-radical synthetic methods for the syntheses of heterocycles.